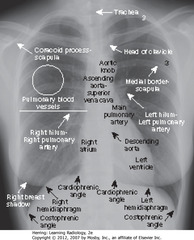
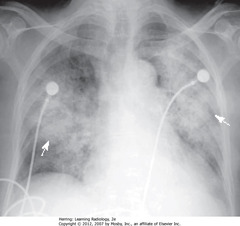
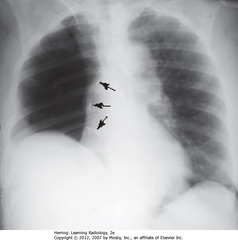
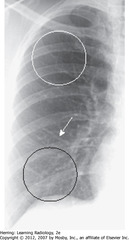
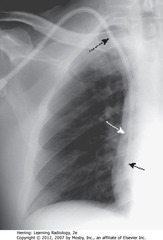
• Spine seen through heart shadow
• R, L lateral costophrenic angles sharply, acutely angled
• WL: level of minor/horizontal fissure, usually visible on frontal view (seen en face); no minor fissure on L side
• WC: lung markings (BVs); L hilum higher
• White”3″: posterior 3rd rib
• Black “3”: anterior third rib
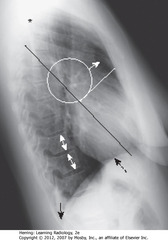
• SWL: clear space behind sternum
• WC: hila produce no discrete shadow
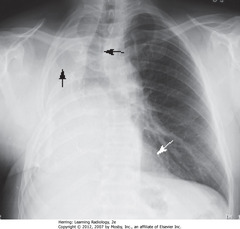
• SWA: Retrosternal clear space
• DWA: vertebral bodies – same height, end plates parallel
• SBA: sharp posterior costophrenic angles
• Black star: thoracic spine blackens (darkens), shoulder girdle to diaphragm (less dense tissue for x-ray beam to traverse at level of diaphragm)
• Heart normally touches anterior aspect of L Hd, usually obscures (silhouettes) it
• DBA: superior surface of R Hd – seen continuously from back to front (not obscured by heart)
• Normal space posterior to heart, anterior to spine (helps assess cardiomegaly)
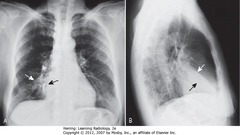
• BL: Major or oblique fissure (visible en face on lateral view)
• WL: horizontal fissure (visible en face on lateral view)
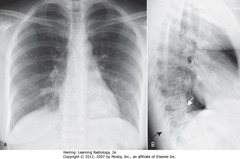
• Spine appears whiter (more dense) above diaphragm (normally gets blacker from neck to diaphragm – less dense tissue for x-ray beam to traverse above diaphragm than in shoulder girdle)
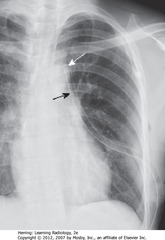
• Airspace disease on lateral film – LLL (frontal – PNA in LLL behind heart)
• WA: LLL PNA superimposed on lower spine on lateral view makes spine whiter (more dense) above diaphragm
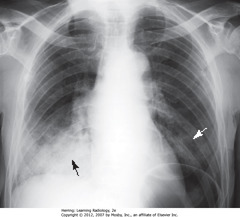
• Lateral projection – R & L posterior ribs almost superimposed
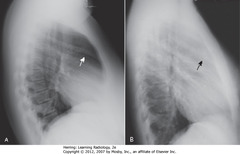
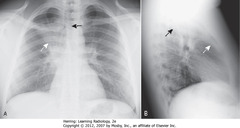
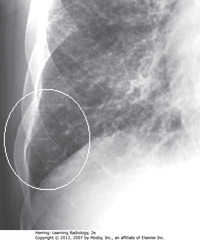
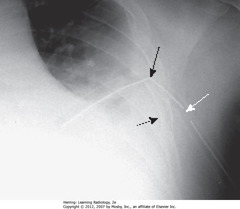
• A: Retrosternal clear space (normal)
• B: soft tissue filling retrosternal clear space behind sternum
• Adenopathy = most frequent reason retrosternal clear space obscured
• Thymoma, teratoma, substernal thyroid enlargement can produce anterior mediastinal masses, but don’t usually produce exactly this appearance
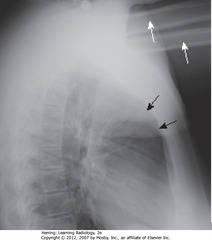
• WA: humeri visible
• BA: soft tissue of arms – not anterior mediastinal adenopathy
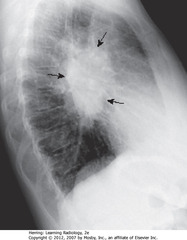
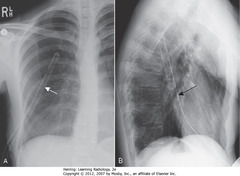
• BA: lobulated mass in hilar region
• Normally, hila don’t cast shadow detectable on lateral projection
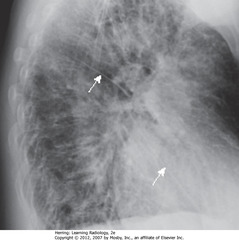
• Thickening of L&R major fissures (WA)
• Major/oblique fissure: 5th thoracic vertebral body to ant diaphragm 2 cm behind sternum
• Dx: CHF; thickening = fluid in fissures
• Fissures usually invisible – if visible, as fine white lines
• Interstitial markings throughout from fluid in interstitium
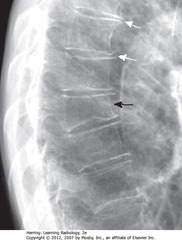
• BA: OP of 8th thor vertebral body – compression fx (often superior endplate first)
• WA: Small osteophytes from degenerative disc dz
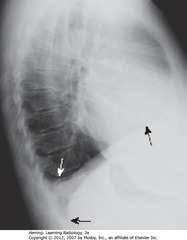
• WA: fluid blunting posterior costophrenic sulcus
• BA: other posterior costophrenic angle is sharp
• DBA: pleural effusion on R (Hd involved can be traced forward anteriorly further than other – left Hd; left Hd normally silhouetted by heart, not visible anteriorly)
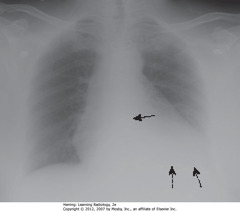
• SBA: spine not visible through cardiac shadow
• DBA: left Hd not visible
• Get lateral CXR to differentiate between artifact of technique & disease
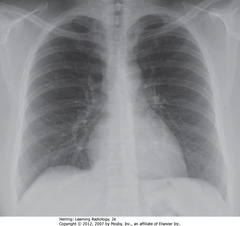
• Lung markings difficult to see – mimics emphysema findings or PTX
• Don’t miss 2nd posterior rib – frequently overlaps 1st rib
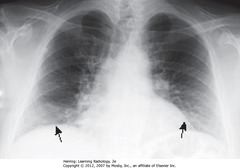
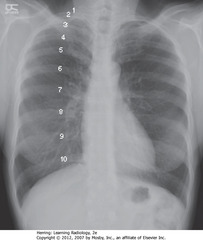
• 8 posterior ribs visible
• Black arrows: crowded – accentuates lung markings at bases
• Crowded lung markings mimic aspiration or PNA
• Get lateral CXR to determine if basilar airspace disease actually present
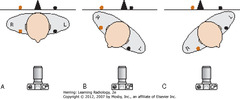
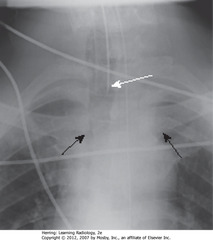
• A: not rotated, medial ends of right (orange dot) and left (black dot) clavicles projected on radiograph (black line) equidistant from spinous process (black triangle)
• B: rotated toward his own right. Medial end of left clavicle (black dot) projected closer to spinous process than is medial end of right clavicle (orange dot)
• C: rotated toward his own left. Medial end of right clavicle (orange dot) projected closer to spinous process than medial end of left clavicle (black dot)
• Same relationships would be on PA projection
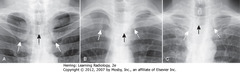
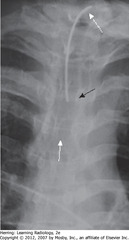
• A: White arrows: clavicles equidistant from spinous process (black arrows) – not rotated
• B: rotated toward his own right (viewing study as if patient facing you; spinous process (BA) much closer to left clavicular head (DWA) than to right clavicular head (SWA)
• C: rotated toward his own left. Spinous process (BA) much closer to right clavicular head (SWA) than to left (DWA)
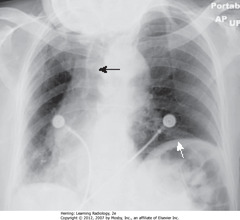
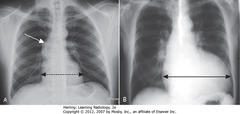
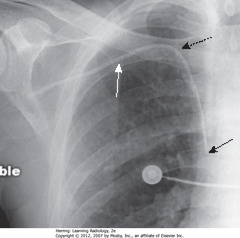
• Pt rotated toward her right
• WA: left Hd appears higher (farther from cassette than right – rotation)
• BA: heart, trachea (arrow) displaced into right Ht (rotation)
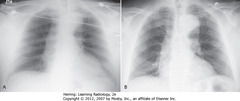
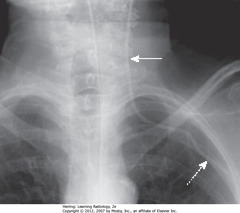
• A: AP projection
• B: PA projection – same patient
• Usually little difference if equal inspiration taken on both
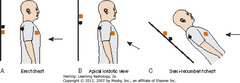
• A: beam correctly oriented perpendicular to cassette plane
• B: beam angled upward – apical lordotic view, anterior structures projected higher than posterior
• C: Semirecumbant study – same result as B (anterior structures higher)
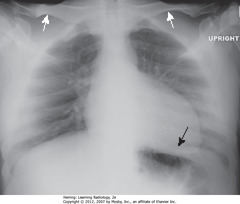
• Most frequently inadvertent – patients semirecumbant
• WA: clavicles projected above first ribs, normal “S” shape is straight
• BA: left Hd obscured, heart shape distorted
• Fluffy, hazy, cloudlike opacities through both lungs, mainly in upper lobes
• Pulmonary alveolar edema
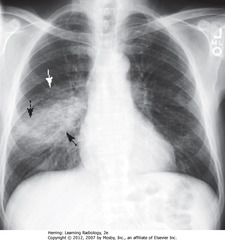
• BA: increased opacification in right midlung field
• WA: indistinct margins, characteristic of airspace disease
• DBA: minor fissure bisects disease – PNA in superior segment of RLL
• RH border, R Hd visible (disease not in contact)
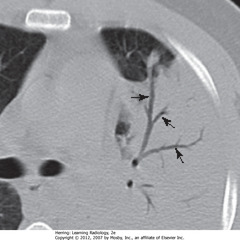
• Black arrows: black, branching structures = air visible in bronchi because surrounding airspaces filled with inflammatory exudate
• Dx: obstructive PNA from bronchogenic carcinoma
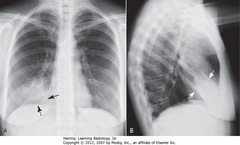
• Fluffy, indistinctly marginated airspace disease right of heart
• Right heart border obscured (SBA)
• Right Hd not obscured (DBA)
• Silhouette sign: obscuring – means disease touching border, same radiographic density as heart
• PNA fills airspaces w/inflammatory exudate of fluid density
• Major fissure – below PNA (DWA)
• Minor fissure – above PNA
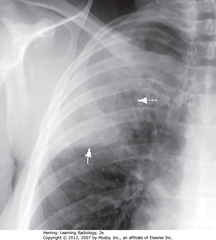
• DWA: air bronchograms
• SWA: minor fissure
• Inferior margin of PNA more sharp because in contact w/minor fissure
• Streptococcus pneumoniae
• WA: bat-wing – fluffy, bilateral, perihilar airspace dz, indistinct margins
• No air bronchograms
• Heart enlarged
• Fluffy, confluent, indistinct margins = airspace dz (BA)
• Similar density in LLL (WA)
• Bibasilar distribution – suspect aspiration
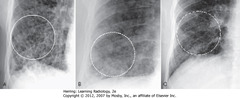
• A: RETICULAR – crisscrossing lines (dx: advanced sarcoidosis)
• B: NODULAR (dx: small metastatic foci from thyroid carcinoma)
• C: RETICULONODULAR – most interstitial diseases- lines and dots (dx: sarcoidosis)
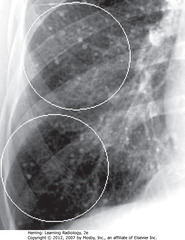
• WCs: small, discrete nodules in R lung – calcified granulomas in lung interstitium
• Clears w/multiple small calcified granulomas remaining
• SBA: part confluent, like airspace
• BC: reticular interstitial dz at periphery
• Look at margins of parenchymal lung dz to determine nature of areas of abnormality & differentiate airspace from interstitial disease
• BC: accentuated pulmonary interstitial markings
• WC: Kerley B lines – fluid in thickened interlobular septa
• BA: fluid in inferior accessory fissure
• A: (BC) coarse reticular interstitial markings of fibrosis, predominantly at bases
• B: (BC) honeycombing – small cystic spaces at subpleural bases – typical distribution for UIP (usual interstitial PNA)
• WA: ground glass opacities (hazy densities)
• IPF = disease spectrum – may begin as desquaminative interstitial PNA (DIP) and lead to UIP
• Reticular markings at bases (WAs)
• MC manifestation rheumatoid lung dz: pleural effusion
• 2nd MC manifestation rheumatoid lung dz: pulmonary fibrosis – usually diffuse but more prominent at bases
• Bibasilar interstitial dz also in bronchiectasis, asbestosis, desquaminative interstitial pneumonia (DIP), scleroderma, SCD
• WA: mass in RUL
• BA: margin slightly indistinct along superolateral border
• MC presentation adenocarcinoma of lung: peripheral nodule
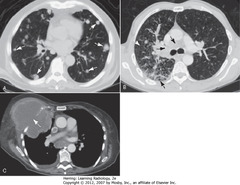
• A-WA: – multiple discrete nodules, varying size, throughout both lungs
• Dx of exclusion when multiple nodules in lung is metastatic dz
• B-WA: prominent interstitial markings
• B-DBA: septal lines
• B-SBA: lymphadenopathy from lymphangitic spread bronchogenic carcinoma
• C-WA: lung CA grown through chest wall, invaded by direct extension
• Pleura usually strong barrier to direct tumor spread
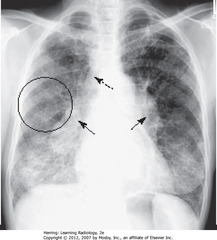
• SBA: bilateral hilar adenopathy
• DBA: right paratracheal adenopathy
• BC: bilateral reticulonodular interstitial lung dz
• Classic distribution for adenopathy in sarcoidosis
• Adenopathy may regress, interstitial dz remains
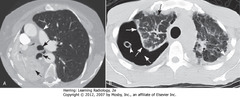
• A-SBA: entire right lung atelectasis (obstructing endobronchial lesion); visceral, parietal pleura in contact
• A-DBA: trachea and main right bronchus shift toward atelectasis
• A-SWA: left lung overexpands, crosses midline
• B: large right PTX
• B-SWA: air interposes between visceral and parietal pleurae (dotted white arrows)
• B-SBA: passive atelectasis
• B (arrowhead): chest tube, removed from suction
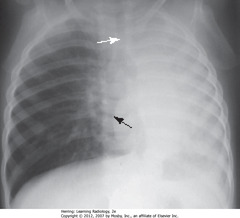
• Heart shifted toward atelectasis (L), overlies spine (BA)
• Trachea L from midline, toward atelectasis (WA)
• Dx: child w/asthma, mucous plug obstructing L main bronchus
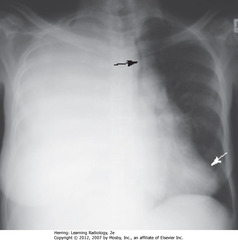
• Black arrow: trachea to L, apex of heart to L
• White arrow: apex of heart – displaced to L, close to lateral chest wall
• Thoracentesis removed 2L serosanguinous fluid
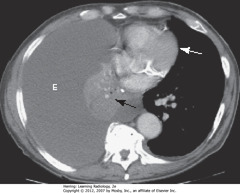
• E: large PLEURAL EFFUSION
• BA: atelectasis of right lung
• Balance between atelectasis and effusion = no shift of midline structures
• WA: heart in normal position
• Strongly suggestive of central bronchogenic malignancy w/malignant effusion
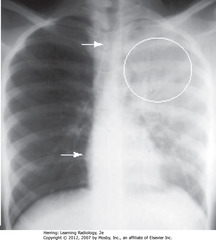
• No heart shift, some trachea shift (WA)
• Air bronchograms w/in upper area of opacification (WC)
• Suggestive of PNA rather than atelectasis or pleural effusion
• Dx: S. pneumoniae of LUL
• <24h after pneumonectomy • BAs: surgical clips in area of R hilum • R 5th rib removed • Over several weeks, R hemithorax fills w/fluid, structures shift toward side of pneumonectomy
• SBA: R 5th rib absent
• WA: heart deviated toward opacification
• DBA: trachea deviated toward opacification
• Deviation towards opacification = volume loss
• Fluid filling R Ht after pneumonectomy fibrosed? permanent shift toward pneumonectomized side
• A-SWA: increased density
• A-SBA: R heart border, silhouetted by density – indicates anterior RML location
• B-DWA: minor fissure displaced downward
• B-DBA: major fissure displaced upward
• Anterior middle lobe
• A: Fan-shaped increased density = airless RUL
• A-SWA: minor fissure displaced up
• A-SBA: trachea shifted to R
• B: similar wedge-shaped density near lung apex
• B-SWA: minor fissure – pulled upward
• B-SBA: major fissure – pulled forward
• Dx: child w/asthma, mucus plug obstructing RUL bronchus
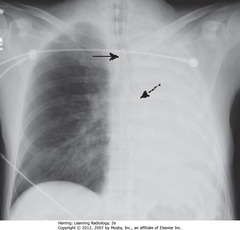
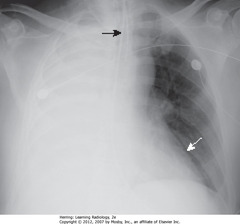
• Complete L Ht opacification
• SBA: trachea – shifted to L (side of atelectasis)
• DBA: NGT in esophagus – shifted to L (side of atelectasis)
• RH border pulled to L side, not visible
• Dx: obstructing bronchogenic carcinoma of L main bronchus
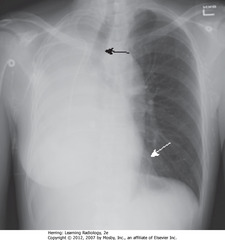
• Complete R Ht opacification
• SBA: trachea shifted toward atelectasis
• SWA: LH border displaced to right, almost overlaps spine
• Dx: endobronchial metastasis in R main bronchus from L-sided breast CA
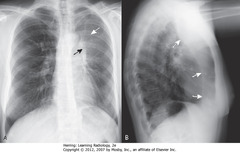
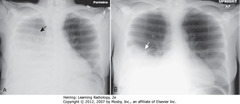
• Hazy density around L hilum (A-SWA)
• Soft tissue mass in L hilum (A-SBA)
• A: L Hd pulled up to same level as right
• B – SWA: bandlike zone of increased density – atelectatic LUL, sharply demarcated by major fissure (pulled anterior)
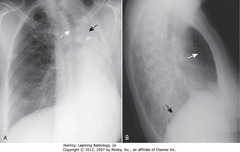
• A: complete opacification of L Ht – fibrothorax after removal; volume loss
• A – SWA: trachea shifted to L
• A – SBA: L 5th rib removed
• A: R lung herniated across midline to fill in L Ht
• B – SWA: increased lucency behind sternum (R lung filling in L Ht)
• B – SBA: only R Hd visible on lateral projection, because only R Ht has aerated lung
• L Hd silhouetted by airless Ht above it
• SBAs: Linear densities extending across all segments of LLs, parallel to diaphragm
• Characteristic of subsegmental atelectasis, aka discoid or platelike atelectasis
• Dx: insufficient inspiration after surgery – resolved on its own
• Passive compression: poor inspiratory effort (A) or 2° large pleural effusion or PTX (B)
• A – SWA: increased density at bases – poor inspiratory effort
• B – SBA: large pleural effusion
• B – DBA: atelectatic LLL, compressed by surrounding pleural fluid
• SBA: no shift of trachea
• SWA: no heart shift
• Absence of shift ? possibility of atelectasis and pleural effusion in balance
• Complete opacification of right Ht
• No air bronchograms to suggest PNA
• Suspect central bronchogenic carcinoma (obstructive atelectasis) with metastases (pleural effusion)
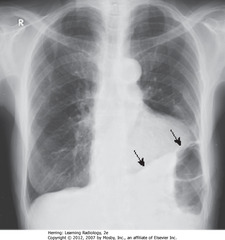
• Masslike density in LLL (CBA)
• Pleural plaques from asbestos exposure (SBA)
• Comet-tail shaped bronchovascular markings from mass extending back into hilum (SWA)
• Combination characteristic of round atelectasis – don’t mistake for tumor
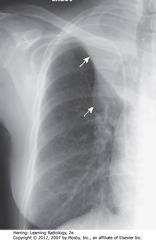
S SIGN OF GOLDEN
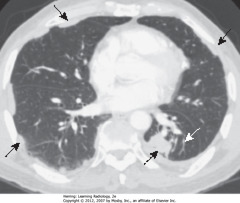
• SWA: soft tissue mass in R hilum
• Opacification of RUL from atelectasis
• DWA: minor fissure displaced up, toward inc density ? RUL volume loss
• S Sign of Golden: Shape of curved edge formed by mass + elevated minor fissure
• Dx: large squamous cell carcinoma obstructing RUL bronchus
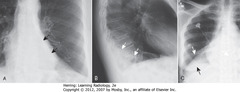
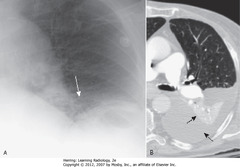
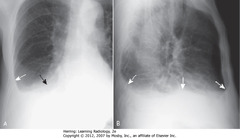
• A – SBAs: fan-shaped increased density behind heart, sharply demarcated by medially-displaced major fissure = characteristic of LLL atelectasis
• B – SWA: major fissure, displaced posteriorly
• Small triangular density in posterior costophrenic sulcus = characteristic location for LLL atelectasis on lateral film
• C – SWA: major fissure – superior border of fan-shaped triangular density in RLL
• C – SBA: unaerated LL silhouettes R Hd
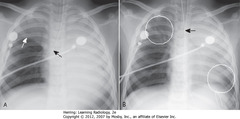
• Tip is past carina into bronchus intermedius – aerates RML, RLL (A-SBA)
• A: RUL & L lung opaque from atelectasis
• Elevated minor fissure (A-SWA)
• ETT retracted above carina (B-SBA)
• RUL, part of LLL aerated again (B-WCs)
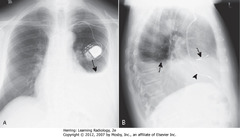
• SBAs: L pleural effusion
• 2-3w after transmural MI or pericardiotomy (CABG)
• CP + fever + L pleural effusion + patchy LLL airspace disease + pericardial effusion several weeks post-MI or CABG
• DBAs: pacer leads in RA
• Arrowhead: pacer leads in RV
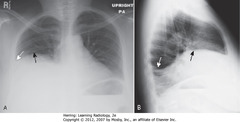
• Elevated R Hd – actual R Hd blocked by pleural fluid over it (A-SBA)
• Blunted right costophrenic sulcus (A-SWA)
• Blunted posterior costophrenic sulcus (B-SWA)
• Apparent Hd rounded posteriorly, but changes contour as effusion interfaces with L side major fissure (B-SBA)
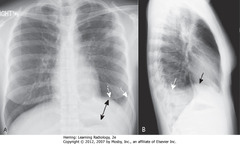
• >1 cm between air in stomach and apparent L Hd (A-DBA)
• A: Edge btwn aerated lung & dotted white arrow isn’t actual L Hd (L Hd invisible due to pleural fluid above), but interface btwn effusion & lung base
• Blunted left costophrenic sulcus (SWAs)
• Apparent Hd rounded posteriorly, but changes contour as effusion interfaces w/major fissure (B-SBA)
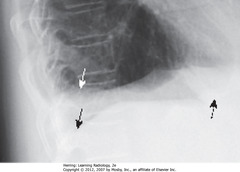
• Posterior CP sulcus blunted first, w/75 mL fl in pleural space (SWA)
• Normal, sharp posterior CP angle visible opposite side (SBA)
• Normal L Hd silhouetted by heart anteriorly, indicating it’s L Hd (DBA)
• Pleural effusion on R side
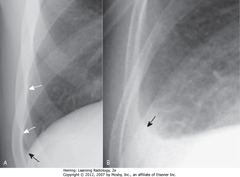
• A – SBA: lateral costophrenic angle created when Hd makes sharp, acute angle as it meets lateral chest wall on frontal
• A – SWA: normally aerated lung extends to inner margin of each rib
• B – SBA: lateral costophrenic sulcus loses acute angulation, becomes blunted when effusion reaches 300 mL volume
• SBA: “Ski slope appearance” – blunting from scars (infection, surgery, blood in pleural space)
• Fibrosis won’t change in appearance or location with change in patient position
• Effusion usually ascends more laterally than medially (A-SBA) on frontal because of natural elastic recoil (A-SWA)
• Lateral – fluid ascends same amount anteriorly & posteriorly, forming U-shaped, meniscoid density (B-SWA)
• A – SBA: Recumbent – R-sided effusion layers along posterior pleural surface, produces “haze” over entire hemithorax, densest at base, less dense toward apex of lung
• B – SWA: Same pt, few minutes later in upright position – pleural fluid falls to base of thoracic cavity from gravity
• X-ray in same position each time
• A: Right lateral decubitus – horizontal beam PA
• A – SBA: free-flowing pleural fluid layers along right side – bandlike density
• A – DBA: Fluid in minor fissure
• B: Left lateral decubitus (same pt)
• B – SBA: Free fluid on left side layers along left lateral chest wall
• Dx: pleural effusions from lymphoma
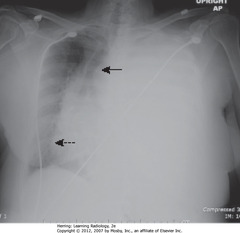
• Left Ht completely opacified
• SBA: trachea – shifted away from opacification
• DBA: heart – shifted away from opacification
• Shifting away = characteristic of large pleural effusion – acts like mass
• Adults: 2L fluid required to fill/almost fill entire Ht
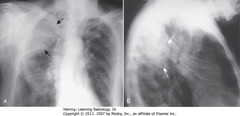
• A – SBA: pleural-based soft tissue density in RU lung field = loculated pleural effusion
• B – SWA: pleural-based soft tissue density in RU lung field = loculated pleural effusion
• Suspect loculated effusion when effusion has unusual shape or location in thorax (ex. effusion remains at apex even if pt upright)
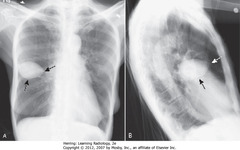
• Sharply marginated pleural fluid collection between layers of minor fissure produce characteristic lenticular shape
• Pointed ends where insinuate into fissure, so pseudotumors look like lemon
• Almost always in CHF pts, disappearing w/CHF treatment, reappeas when recurs
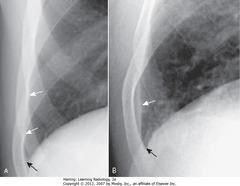
• Normal – normally aerated lung extends to inner margin of ribs (A-SWA)
• Sharp costophrenic sulcus (A-CBA)
• Thin band of increased density extends superiorly from base, but doesn’t blunt costophrenic angle = laminar pleural effusion (B-SWA)
• Costophrenic angle (B-SBA)
• Laminar pleural effusion most often associated with CHF or lymphangitic spread of malignancy in lung
• Dx: CHF
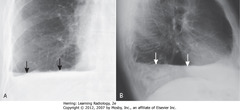
• A-SBA, B-SWA: air-fluid level in Ht – marked by straight edge; sharp, air-over-fluid interface (horizontal beam)
• Causes: surgery, trauma, recent thoracentesis, bronchopleural fistulae
• Dx: R-side stab wound – actually a hemopneumothorax, but can’t distinguish on CXR between blood & other fluid
• Pleural tap to define pleural fluid
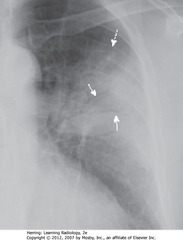
• Air bronchograms – seen centrally in airspace disease (SWA)
• Homogeneous density except air bronchograms
• Outer edges fluffy, poorly marginated (characteristic of airspace disease) (DWA)
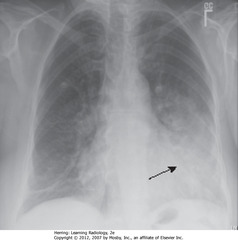
• Airspace in lingular segments of LUL
• Homogeneous density
• SBA: L lateral border of heart silhouetted by fluid density of consolidated UL with heart
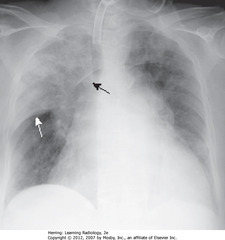
• Airspace disease in entire RUL
• SWA: Minor/horizontal fissure makes sharp margin on inferior aspect of PNA
• SBA: border of ascending aorta where fluid-density of PNA silhouettes it
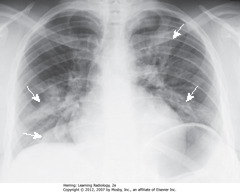
• Irr. marginated patches airspace disease, both lungs – typical bronchopneumonia distribution/appearance (SWA)
• Spreads from tracheobronchial tree to multiple foci in lung (often involves several segments)
• Lung segments not bound by fissures – so margins of segmental PNAs are fluffy, indistinct
• No air bronchograms – inflammatory exudate fills bronchi and surrounding airspaces
• Diffuse interstitial, primarily reticular, lung dz
• No pleural effusions (would be in pulmonary interstitial edema
• No evidence of sarcoidosis (no hilar adenopathy)
• Pt has hx AIDS
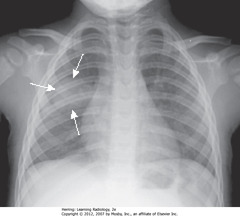
• SWA: soft tissue density, round appearance, right midlung field, characteristic of round PNA
• MC in children, often from Haemophilus, streptococcal, or pneumococcal infxn
• Pt – infant
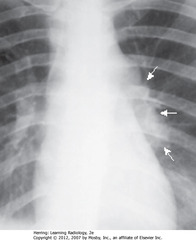
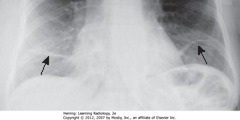
• SWA: prominence of L hilum from L hilar adenopathy
• Primary TB affects UL slightly more than LLs
• Airspace disease can be associated w/ipsilateral hilar adenopathy (esp. in children) and large, usually unilateral pleural effusions (esp. in adults)
• Unilateral hilar adenopathy can be only manifestation of primary infection with Mycobacterium tuberculosis
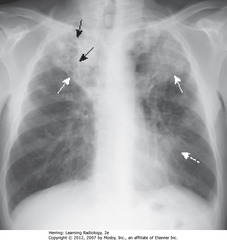
• SWA: cavitary PNA ULs
• SBA: Lucencies (cavities) throughout airspace dz in RUL
• Cavitary UL PNA presumed TB until proven otherwise
• DWA: Airspace disease in lingula
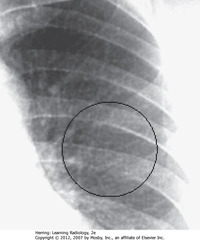
• Multiple small nodules in L lung (BC)
• Visible when 1 mm or larger
• Miliary TB will clear quickly with appropriate tx, doesn’t calcify
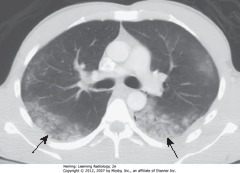
• SBA: LL airspace dz in pt that aspirated
• Affects most dependent parts of lung – LLs if upright, superior LLs and posterior ULs most involved
• Usually clears w/in 24-48 hours
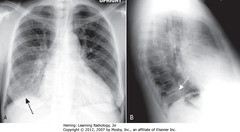
• SBA: RLL airspace disease
• Thoracic spine usually gets blacker from neck to diaphragm
• SWA: RLL PNA superimposed on lower spine makes spine appear whiter (more dense) above diaphragm (spine sign)
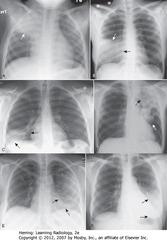
• A:RUL – sharp margin PNA obscures (silhouettes) ascending aorta (RA)
• B: RML – disease silhouettes R heart border (BA)
• C: disease silhouettes right Hd (BA)
• C: R heart border (DBA)
• D: LUL – dz poorly marginated (WA)
• D: obscured aortic knob (BA)
• E : dz silhouettes L heart border (SBA)
• E: L Hd (DBA)
• F: disease obscures L Hd (DBA)
• F: L heart border (SBA)
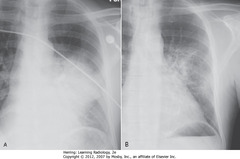
• PNA, esp. pneumococcal, can resolve in 2-3d if sensitive to abx given
• A & B taken 4 days apart
• Vacuolize (resolve from within), disappear in patchy fashion over days-weeks
• Consider neoplasm if present more than a few weeks, preventing drainage
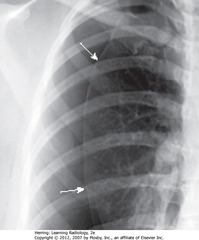
• SWA: visceral pleural line
• Air in pleural space ? pleura retracts to hilum along with collapsing lung
• Visceral pleura visible as thin, white line with air outlining both sides
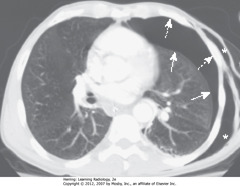
• SWA: Curve of visceral pleural line
• DWA: Curve of chest wall
• Visceral pleural line parallel to curve of chest wall differentiates PTX from artifacts, other diseases that mimic PTX
• Lung on side of PTX remains lucent until it loses almost all its normal volume, then appears opaque
• White stars: subcutaneous emphysema (air in soft tissues) of L lateral chest wall
• Dx: stab wound
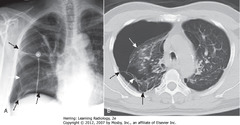
• Lung markings seen distal to visceral pleural line, if pleural adhesions present on CXR
• Pleural adhesions keep PTX from collapsing lung (SWA)
• Pleural adhesions (A-SBA)
• Pleural adhesions holding lung against parietal pleural (B-SBA)
• Lung (B-SWA)
• Adhesions MC due to prior infection or blood in pleural space
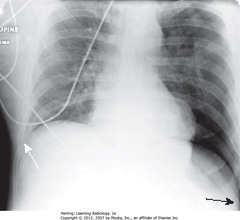
• SBA: When supine, air in large PTX collects anteriorly/inferiorly in thorax, manifests by displacing costophrenic sulcus inferiorly, while producing increased lucency of that sulcus (deep sulcus sign)
• Sign of PTX on supine CXR
• SWA: right sulcus – not as low as L costophrenic sulcus
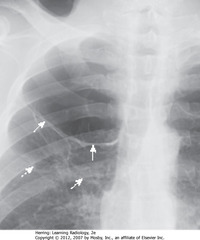
• SWA: white line in RUL, no lung markings peripheral to it
• Line is convex away from chest wall, doesn’t parallel chest wall curve
• Classic appearance of bulla of emphysema
• Placing CT into bulla almost always causes PTX that can be hard to re-expand
• DWA: walls of several bullae
• Vanishing lung syndrome: bullae so large that Ht seems devoid of visible lung tissue
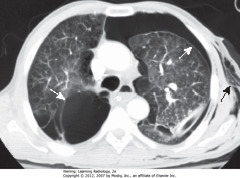
• Axial CT section
• DWA: bullous disease with border convex away from chest wall
• SWA: PTX with border convex toward and paralleling chest wall
• SBA: subcutaneous emphysema
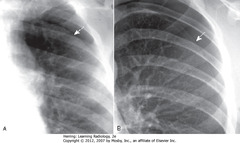
• A – DWA: skin fold in expected position of PTX – may parallel CW as chest wall (Left image)
• B – SWA: PTX – thin white line of visceral pleura
• Skin folds produce thicker, white bands of density
• Skin fold = edge; visceral pleura = line
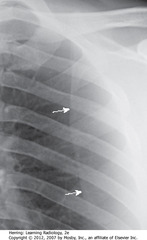
• SWA: medial border of scapula, superimposed on upper lung field – may mimic PTX
• Identify medial border of scapula before diagnosing PTX
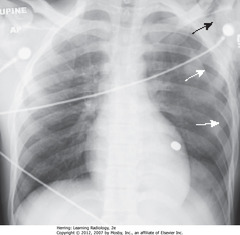
• SWA: Large L PTX – with no shift of heart or trachea to right
• SBA: SQ emphysema in region of left shoulder
• Bullet superimposed on heart
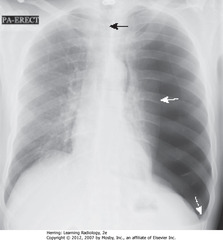
• SWA: L lung almost totally compressed
• SBA: trachea; trachea and heart shifted to right
• DWA: L Hd depressed due to increased L intrathoracic pressure
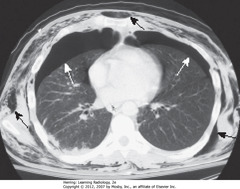
• Smaller PTX may only be visible on chest CT
• SWA: bilateral pneumothoraces
• Air rises to highest point (pt supin in CT)
• SBA: extensive subcutaneous emphysema, developed from air leak from chest tube inserted earlier
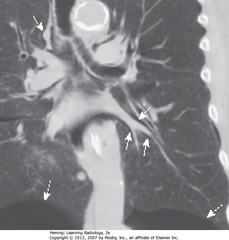
• SWA: air surrounding pulmonary arteries
• Air from ruptured alveolus in asthma pt; tracked back to hilum, produced pneumomediastinum and SQ emphysema
• DWA: bilateral, basilar PTXs
• SWA: streaky white densities extending to neck – produced by air from pneumomediastinum tracking back to hila, then into mediastinum
• DBA: SQ emphysema in neck
• SBA: pneumopericardium – unusual in adults – air usually only enters pericardium by direct penetration
• Air in pericardial space isn’t extending above reflections of aorta and pulmonary artery
• Pneumomediastinum does extend above great vessels, normally
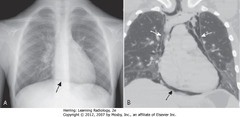
• A – SBA: air outlines central part of diaphragm under heart -> unbroken diaphragmatic contour extending from one lateral chest wall to other = continuous diaphragm sign
• Diaphragm not normally visible in center of chest because no air in mediastinum and soft tissue density of heart rests upon and silhouettes soft tissue density of diaphragm
• B – SBA: pneumomediastinum ourlining central portion of diaphragm
• B – SWA: remainder of pneumomediastinum extending superior to great vessels
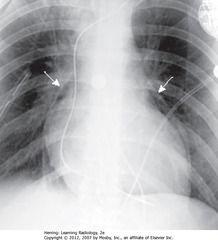
• SWA: visible parietal percardium outlining air around heart in pericardial space – indicates pneumopericardium
• Air does not extend above reflection of aorta and main pulmonary artery
• Pneumopericardium in adults usually from trauma
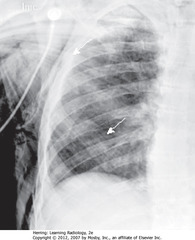
• SWA: air dissecting along muscle bundles looks comblike, striated
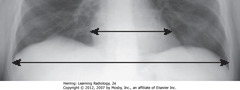
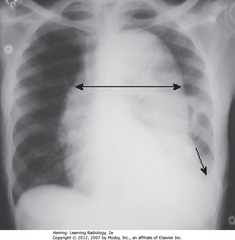
• UDA: widest diameter of heart
• LDA: widest internal diameter of thoracic cage from inside rib to inside rib – usually at diaphragm level
• Cardiothoracic ratio should be <50% in normal adults
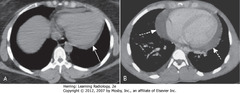
• A – SWA: fluid accumulating in dependent portions of pericardial space, posterior to LV in supine position
• B – DWA: larger effusion fills pericardial space, encircles heart
• CXR may show enlarged cardiac silhouette but can’t differentiate density of ehart from effusion
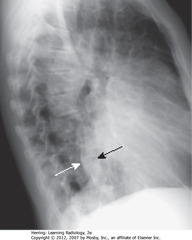
• Post heart border overlapping thoracic spine
• Lateral projection – confirm enlargement
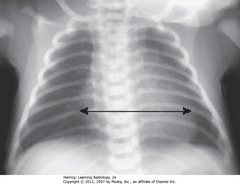
• Cardiothoracic ratio up to 65%
• Assessment of cardiac enlargement in infant should consider other factors such as appearance of pulmonary vasculature, associated S/S (such as a murmur, tachycardia, or cyanosis)
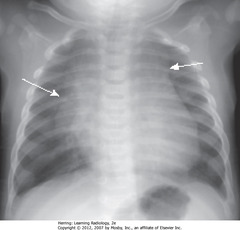
• May overlap upper portion of the cardiac silhouette
• Can be mistaken for cardiomegaly in a child
• SWA: thymus often lobulated in appearance
• Usually involves by 3y, may be visible up to 8y
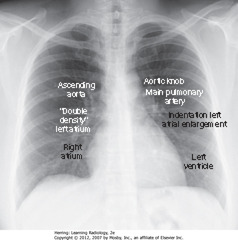
• 7 identifiable cardiac contours on the frontal chest radiograph.
1. R side of heart: first contour = low-density, almost straight edge visible just lateral to trachea, reflecting size of ascending aorta
2. Slight indentation where contour of ascending aorta meets contour of RA, where LA may appear when it enlarges
3. RH border formed by RA
4. First left contour = aortic knob, formed by foreshortened aortic arch superimposed on part of proximal descending aorta
5. Next contour below aortic knob is main pulmonary artery, before it divides into R & L pulmonary artery
6. Just below main pulmonary artery segment, normally a slight indentation where enlarged LA may appear on L side of heart
7. LV forms last contour of heart on the left
• Descending aorta almost disappears with shadow of spine
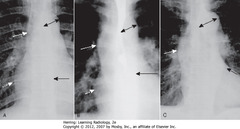
A: Normal
• SWA: ascending aorta – low-density, almost straight edge, doesn’t project beyond R heart border
• DWA: R heart border
• DA – normal aortic knob size
• SBA: descending aorta, almost disappears with shadow of thoracic spin
B: AORTIC STENOSIS (ascending Ao to R)
• SWA: ascending aorta projects almost to RH border (DWA) – dilated from stenosis
• DA: normal aortic knob
• SBA: normal descending aorta
C: HTN (enlarged aortic knob)
• SWA: ascending aorta – too right
• SBA: descending aorta – too left
• DA: enlarged aortic knob
• BC: LL vessels – larger in size than UL vessels in upright position
• WC: UL vessels
• WA: vessels all taper gradually from central to peripheral
• A – WA: poststenotic dilatation of ascending aorta present – aortic stenosis
• A – DBDA: heart not enlarged (lesion produces LVH
• Dx: aortic regurgitation
• B – SBDA: enlarged LV
• White oval: Kerley B lines – 1-2 cm long, 1 mm thick, horizontal lines perpendicular to, abutting pleural surface
• Interlobular septae not visible on normal CXR but become visible with fluid accumulation
• Circle: A lines appear when connective tissue near bronchoarterial bundle distends w/fluid
• Extend several cm from hila in midlung, don’t reach periphery of lung like Kerley B lines
• Network of Kerley lines produced in lungs in CHF, producing “prominence of pulmonary interstitial markings”
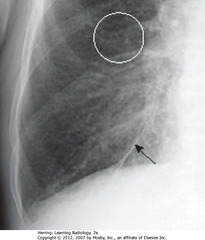
• Bronchus normally invisible when seen on-end in lung periphery
• SWA: bronchial walls, thickened, ringlike densities when seen on-end, caused by fluid accumulation in interstitial tissue around and in wall of a bronchus
• Seen in CHF
• May not always produce perfectly round circles
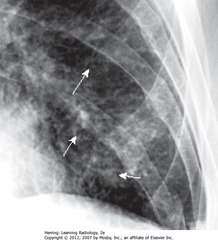
• A – SWA: major fissure
• A – DWA: minor fissure
• B – SWA: major fissure – more prominent (fluid collection distends in CHF)
• B – DWA: minor fissure – more prominent (fluid collection distends in CHF)
• When HF clears, returns to normal, but may fibrose if recurs, causing permanent thickening
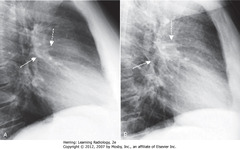
• SBA: Thickened inferior accessory fissure – separates medial from other basilar segments of LL; usually not easily seen
• Circle: peribronchial thickening
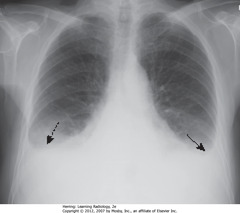
• BAs: bilateral pleural effusions
• Usually bilateral in CHF, may be symmetric, w/right side slightly larger
• Unilateral, L pleural effusion can happen in CHF, but should suspect another cause – ex. metastatic disease
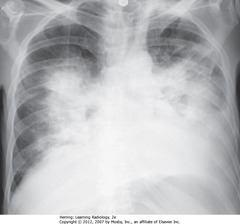
•CXR findings of pulmonary alveolar edema: fluffy, indistinct, patchy airspace densities, often centrally located and not affecting outer third of lung
• Suggests pulmonary edema vs other airspace diseases such as PNA
• Patterns of cardiogenic and noncardiogenic pulmonary edema overlap
• Absence of pleural effusions, absence of fluid in fissures, and normal-sized heart indicate noncardiogenic cause
• Dx: septic shock from an overwhelming urinary tract infection
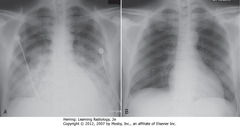
• Onset and clearance of pulmonary edema area fast
• A: Bilateral, perihilar airspace disease, diffuse prominence of interstitial markings characteristic of pulmonary edema
• B: After 4 days, lungs clear
• ARDS, other coexisting diseases – don’t clear as fast
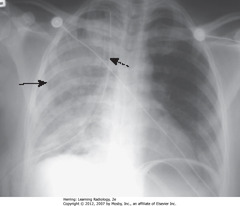
• SBA: unilateral airspace disease affecting entire R lung
• DBA: chest tube for R-sided tension PTX
• Caused by rapid reexpansion of lung that has typically been chronically by PTX or large pleural effusion
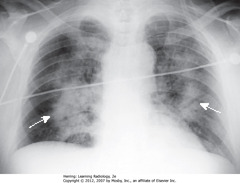
• SWA: Perihilar distribution of airspace disease similar to cardiogenic pulmonary edema
• No pleural fluid, fluid in fissures or cardiomegaly
• Noncardiogenic pulmonary edema less likely to have pleural effusions and Kerley B lines, more likely to have a normal pulmonary capillary wedge pressure (PCWP) of < 12 mm Hg, and more likely to be associated w/normal-sized heart than cardiogenic pulmonary edema
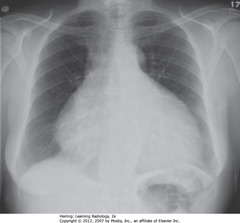
• Hypertrophic cardiomyopathy
• DWA: LV slightly enlarged, but other modalities would demonstrate marked concentric hypertrophy of LV wall that has occurred at the expense of the lumen
• SWA: uncoiled aorta, from increased systemic BP
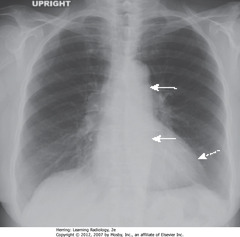
• Enlarged LA (SWA)
• UL vessels more prominent – pulmonary venous HTN redistributing flow in lungs (WC)
• RH changes from PA HTN, leading to TR w/enlarged RA (SBA)
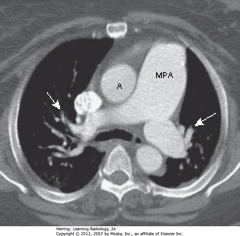
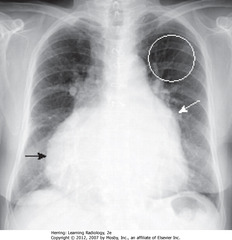
• MPA – main pulmonary artery – usually same diameter as ascending aorta (A)
• This pt has pulmonary artery HTN, so MPA is larger than aorta
• SWA: pruning – rapid attenuation in size of pulmonary arteries seen in pulmonary arterial HTN
• Cardiac silhouette enlarged, mainly from biventricular enlargement
• Frequently associated with CHF
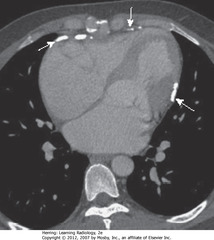
• SWA: pericardial calcification, probably post-inflammatory
• Presence of pericardial calcificications excludes restrictive cardiomyopathy
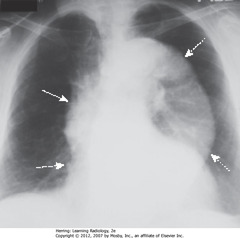
• Entire thoracic aorta enlarged
• Ascending aorta – shouldn’t project farther than RH border (SWA)
• RH border (DWA)
• Descending thoracic aorta normally parallels, almost disappears with thoracic spine
• Enlarged descending thoracic aorta swings farther away from spine (DWA)
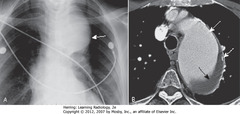
• Large mediastinal ST mass – aneurysm of proximal descending aorta (A-SWA)
• Calcification in aneurysm wall – common (B-DWA)
• Contrast mixing w/blood in aorta lumen (SWA)
• Noncontrast-containing thrombus adhered to wall of aorta (B-BA)
• DBA: widened mediastinum
• SBA: L pleural effusion
• Widened mediastinum + L pleural effusion in pt w/chest pain – signs of aortic dissection
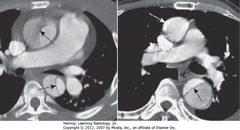
• A – SBAA: intimal flap crossing ascending and descending aorta – Stanford type A dissection
• B – DWA: Normal-appearing asending aorta
• B – DWA: black line – intimal flap crossing descending aorta
• Intimal flap = characteristic of aortic dissection
• Smaller lumen is usually the real lumen, larger lumen is channel made by blood dissecting through the media
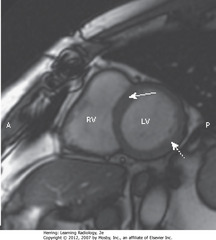
• SWA: Interventricular septum, separating RV and LV
• DWA: wall of LV
• A = anterior, P = posterior
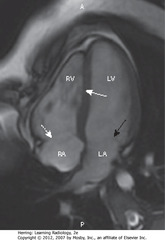
• SWA: interventricular septum separating RV and LV
• Posterior to each – RA and LA
• DWA: tricuspid valve
• SBA: mitral valve
• A = anterior, P = posterior
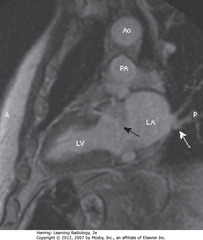
• SBA: MV area separating LA from LV
• SWA: pulmonary veins, drain into LA
• Ao = aorta, PA = pulmonary artery, A = anterior, P = posterior
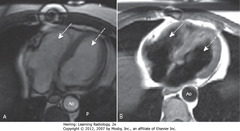
• Both images axial sections through heart
• SWAs = RV
• DWAs = LV
• Ao = aorta
• A = bright blood technique, used to assess cardiac function
• B = black blood technique – used to depict cardiac morphology
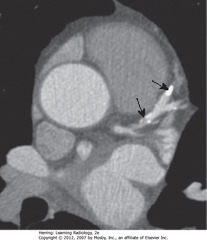
• CT scan of heart w/out IV contrast
• SBAs: calcified plaque in left anterior descending coronary artery
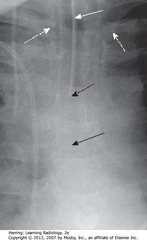
• SWA: radioopaque marker stripe of ETT (usually wide-bore – 1 cm), no side holes
• DBA: diagonal tip of ETT
• SBA: carina – with head in neutral position, ETT tib should be 3-5 cm away (half distance between medial ends of clavicles and carina
• DWAs: medial ends of clavicles
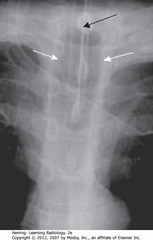
• SBA: ETT – should be 1/3 – ½ width of trachea
• Inflated cuff may fill in but shouldn’t distend tracheal lumen
• SWA: Inflated balloon wider than diameter of trachea
• SWA: tip of ETT – shouldn’t be in larynx or pharynx – should be 3 cm below vocal cords
• SBAs: medial ends of clavicles
• Tip should be halfway between stoma (where inserted) and carina (SBA)
• Tracheostomy (DWA)
• Carina (SWA)
• SWA: CVL – 3 mm – uniformly opaque, no marker stripe
• DBA: medial end of clavicle – CVL should reach here before descending
• SBA: SVC – catheter should descend to right of thoracic spine, with tip in SVC
• DWA: subclavian
• SWA: IJ – CVLs most often malpositioned with tips in RA or IJ
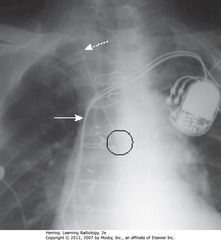
• DWA: medial end of clavicle – catheter doesn’t reach here before descending (it should)
• Black circle: catheter tip, oriented over spine, away from SVC
• SWA: SVC
• Suspect arterial placement if flow is pulsatile
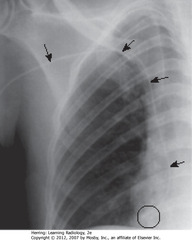
• SBA: PICC line
• Tip should lie within SVC, but may be placed in axillary vein
• BC: tip extends to RA
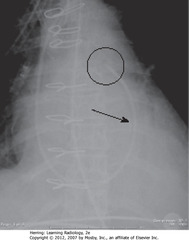
• SBA: Swan-Ganz – same appearance as CVL, but longer
• Inserted via SC or IJ
• BC: tips – floated into proximal R or L pulmonary artery
• Tip should be no more than 2 cm from hilar shadow
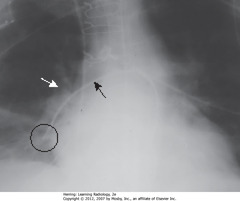
• Catheter directed toward R pulmonary artery (SBA)
• Hilar shadow – tip should be within 2 cm of hilar shadow (SWA)
• Tip of catheter – in peripheral branch of R descending pulmonary artery (BC)
• Could cause pulmonary infarct or pseudoaneurysm
• DBA: central stripe of large-bore catheter
• SWA: tip for blood withdrawal further from heart than tip for blood return
• SBA: tip for blood return
• R IJ most often used for access
• This cath has two, separate, single-lumen caths, one tip in SVC and other in RA
• DBA: side hole of chest tube – stripe breaks at site of side hole
• SWA: ideal position – anterosuperior for evacuating PTX and
• Ideal position for draining effusion = posterior (work regardless of where positioned)
• SWA: side holes outside thoracic wall
• SBA: tube kinked as entering chest
• Underlying pleural effusion
• Air leak makes pleural effusion persist
• A – SWA: tube oriented along course of right major fissure
• B – SBA: tube oriented along course of right major fissure
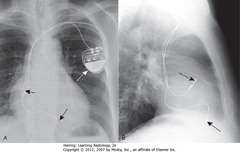
• SWA: pulse generator; usually in L chest wall and 1+ electric leads, usually in SC vein
• SBAs: One lead in apex of RV
• DBAs: One lead in RA
• A – SBA: RV lead projects left of midline
• B – SBA: RV lead projects anteriorly
• RA lead usually curls upward
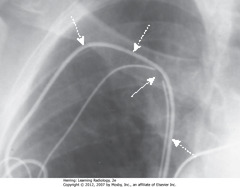
• Fractures usually occur in generator, tip of lead, or site of venous access
• SWA: discontinuity in wire lead in site of venous access to subclavian
• DWA: second, intact lead
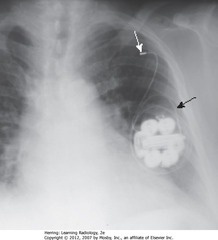
• SBA: generator rotated on its own axis, curling the leads around
• Retracts tip of electrode from inner wall of RV – pacemaker useless
• SWA: L SC vein – lead retracted into
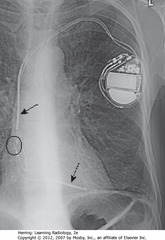
• SBA: electrode in SVC – may also be placed in brachiocephalic vein
• BC: tip of lead in RA
• DBA: electrode tip in apex of RV
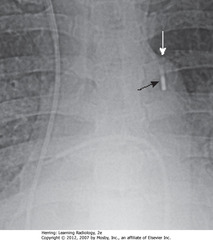
• SBA: small, linear metallic marker of device in region of descending thoracic aorta
• Tip should be distal to LSC artery to not occlude it
• SWA: aortic knob – tip is 1 cm away
• Metallic marker can point slightly toward R in the area of the arch
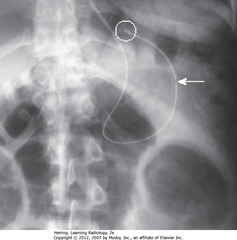
• SWA: tip of NG tube – radiopaque stripe, “breaks” in position of side hole, 1-0 cm from tip
• Tip and all side holes of tube should extend ~10 cm into stomach
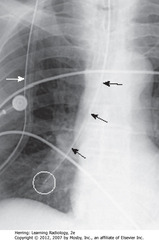
• MC malpositioned of all tubes/lines – usually coiling in esophagus
• SBA: NG tube – entered trachea instead of esophagus
• WC: tip in RLL
• SWA: part of NG tube outside patient is superimposed on chest (so are heart monitor leads)
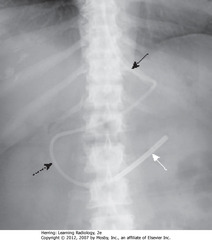
• Tip should be in duodenum
• SWA: weighted, metallic end
• SBA: Dobhoff enters stomach
• DBA: duodenal sweep
• SBA: junction of L Hd and L side of thoracic spine (L cardiophrenic angle) – usual place
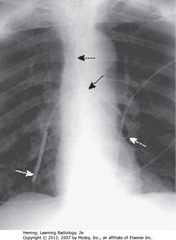
• DBA: Dobhoff enters trachea
• DWA: Dobhoff in LLL bronchi
• SBA: coiled back on itself to cross midline
• SWA: Dobhoff in RLL
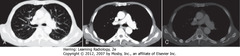
• A – lung window – lung parenchyma, bronchial anatomy
• B – mediastinal window – mediastinal, hilar, pleural structures
• C – bone window
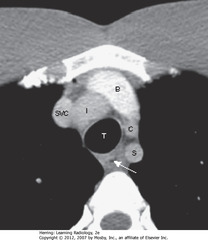
• SWA: esophagus
• Venous structures more anterior than arterial
• SVC to the right of trachea
• Brachiocephalic vein posterior to sternum
• From pt’s right to pt’s left, Inominate artery, left common Carotid, left Subclavian
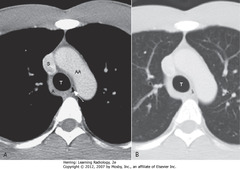
• Aortic arch (AA), superior vena cava (S) and azygous vein (A (T = trachea)
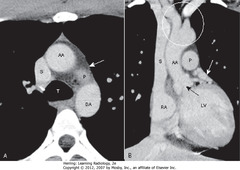
• SWA: aortopulmonary window: usually space visible just under arch of aorta and above pulmonary
• SBA: aortic valve
• SWA: left atrial appendage
• WC: origin of the great vessels
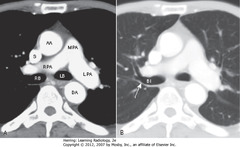
• A: main (MPA), right (RPA), and left pulmonary a (LPA), right (RB) and left main bronchi (LB).
• L pulmonary artery higher than R, appears as continuation of main pulmonary artery
• R pulmonary artery originates at 90° angle to main pulmonary artery, crosses to right side
• B: bronchus intermedius (BI) – distal to the takeoff of the right upper lobe bronchus
• SWA: posterior wall of RUL bronchus is 2-3 mm thick, with only lung posterior to it
• AA = ascending aorta; DA = descending aorta; S = SVC
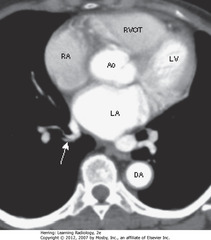
• LA occupies posterior and central portion of heart
• SWA: 1+ pulmonary veins enter LA
• RA produces right heart border, lies anteriorly/to the right of LA
• RVOT: right ventricular outflow tract – lies anterior, lateral and superior to root of the aorta (Ao)
• DA = descending thoracic aorta; LV = left ventricle
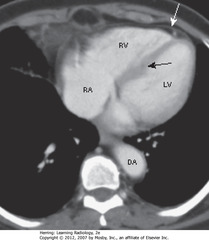
• RA forms right heart border
• RV anteriorly located, just behind sternum
• LV produces left heart border
• SBA: IV septum RV & LV
• SWA: epicardial fat, on inner surface of pericardium; mediastinal fat lines outside of pericardium
• DA = descending thoracic aorta
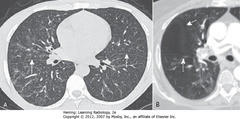
• A – SWA: major fissures – shown as thin white lines or by avascular band 2 cm thick produced as through lungs from posterosuperior to anteroinferior
• B – SWA: Minor fissure in same plane as axial CT – not normally visible, but can be inferred by avascular zone between RUL and RML
• DWA: major fissure
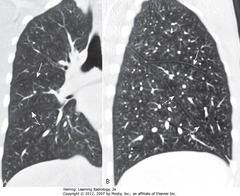
• A – SWA: minor fissure – thin white line on coronal view of RL
• A – DWA: major fissure – travels obliquely image plane, seen as avascular zone
• B – SWA: minor fissure
• B – DWA: major fissure
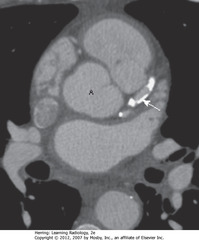
•SWA: dense calcification in L anterior descending coronary artery arising from aorta (A)
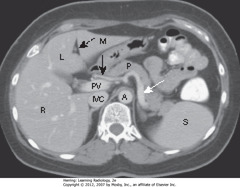
• DBA: ligamentum teres – divides left lobe of liver into medial (M) and lateral segment (L) with larger right lobe (R) lying more posterior
• Portal vein (PV) lies just posterior to hepatic artery
• SWA: splenic artery – follows pancreas (P) toward spleen (S)
• Inferior vena cava (IVC) lies to right of aorta (A)
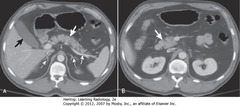
• A – Thick WA: body of pancreas
• A – Thin WA: splenic artery
• A – DWA: adrenal glands
• A – BA: gallbladder
• B – Thick WA: head of pancreas
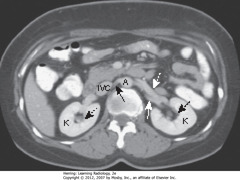
• Kidneys (K) lie in renal fossae bilaterally
• DBA: Normal renal pelvis, containing fat, occupies central portion of the kidneys
• SBA: right renal artery runs posterior to inferior vena cava (IVC)
• DWA: left renal vein lies anterior to left renal artery
• SWA: left renal artery
• A = abdominal aorta
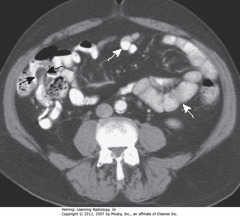
• Contrast fills nondilated lumen of small bowel (< 2.5 cm) • WA: small bowel wall - thin - normally almost invisible • BAs: terminal ileum - recognized by the fat-containing "lips" of ileocecal valve outlined w/contrast
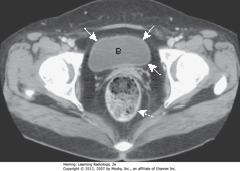
• Urinary bladder (B) with unopacified urine in early image of contrast-enhanced pelvic CT
• SWAs: bladder wall – thin, of equal thickness around the circumference of bladder
• DWA: rectum – posterior to bladder
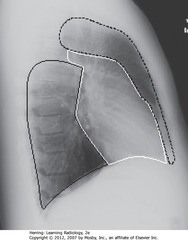
• Three compartments: anterior, middle, and posterior
• DBO: Anterior mediastinum: back of sternum to anterior border of heart and great vessels
• SWO: Middle mediastinum: anterior border of heart and aorta to posterior border of heart and origins of great vessels
• SBO: Posterior mediastinum: posterior border of heart to anterior border of vertebral column (for practical purposes, considered to extend into paravertebral gutters)
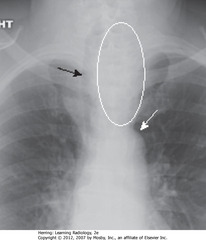
• WO: lower pole of thyroid enlarged, projects downward into upper thorax rather than anteriorly into neck
• SWA: top of aortic arch – substernal thyroid goiters produce mediastinal masses that don’t extend below this
• SBA: trachea, usually displaced by substernal goiters either to left or right above aortic knob (other anterior mediastinal masses don’t do this)
• Think of enlarged substernal thyroid goiter whenever anterior mediastinal mass displaces trachea
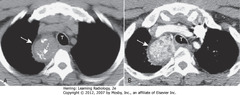
• These are two images at the same level in a patient who was scanned both before (A) and after IV contrast
• SWA: substernal thyroid masses contiguous with thyroid gland
• DWA: calcification in mass
• SWA: avidly take up IV contrast, but w/mottled, inhomogeneous appearance
• Trachea (T) displaced slightly to L
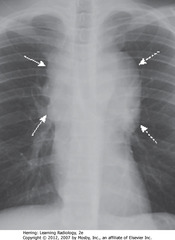
• Lymphadenopathy with lobulated/polycyclic border from enlarged nodes (SWA)
• Finding may help differentiate lymphadenopathy from other mediastinal masses
• DWA: bilateral mediastinal lymphadenopathy, usual in Hodgkin disease is usually bilateral, often asymmetric
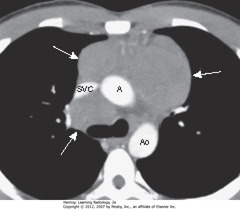
• SWA: lymphomas produce multiple, lobulated soft-tissue masses or a large soft-tissue mass from node aggregation
• Mass usually homogeneous in density, but may be heterogeneous when nodes large enough to undergo necrosis (areas of low attenuation, i.e., blacker) or hemorrhage (areas of high attenuation, i.e., whiter)
• SVC compressed by nodes, ascending (A) and descending aorta (Ao) typically less so
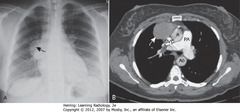
• Thymomas: neoplasms of thymic epithelium & lymphocytes, MC in middle-aged, usually older age than teratoma
• A – SBA: smoothly marginated anterior mediastinal mass
• B – SWA: Contrast-enhanced CT confirms anterior mediastinal location, homogeneous density of mass
• Dx: myasthenia gravis, improved following resection of thymoma
• A = ascending aorta; Ao = descending aorta; PA = main pulmonary artery; SVC = superior vena cava
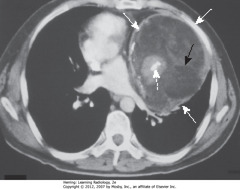
• Teratomas: germinal tumors, typically contain all 3 germ layers
• Often discovered at younger age than thymomas
• SWA: cystic = MC variety
• Usually produce well-marginated mass near origin of great vessels
• SBA: fat
• DWA: bone
• On CT, usually contain fat, cartilage, sometimes bone
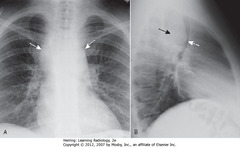
• A – SWA: mediastinal mass
• B – SBA: mediastinal mass
• B – DWA: mass pushing trachea forward
• Lymphoma = MCC adenopathy in middle mediastinum
• Also produced by other malignancies (small cell lung carcinoma, metastatic disease, several benign diseases)
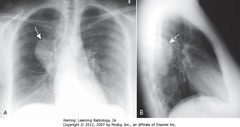
• Can occur as isolated tumor arising from Schwann cell of nerve sheath or as part of syndrome neurofibromatosis
• SWAs: large, posterior mediastinal neurofibroma in right paravertebral gutter
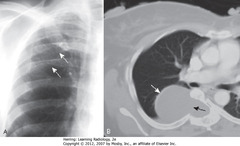
• Ribbon Ribs – notching/wavy appearance from erosions along inferior rib borders produced by plexiform neurofibromas (A-SWA)
• Neurofibroma enlarging neural foramen, eroding half of vertebral body (B-SBA)
• DWA: dumbbell-shaped lesion arising from spinal canal but projects through foramen into mediastinum
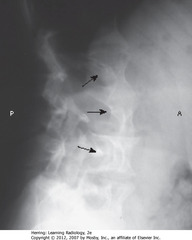
• Neurocutaneous disorder associated with skeletal dysplasia
• May be numerous associated skeletal abnormalities
• SBA: scalloping of posterior vertebral bodies – especially in thoracic or lumbar spine
• Produced by diverticula of thecal sac, caused by dysplasia of meninges ? erosion of adjacent bone through pulsations transmitted via spinal fluid
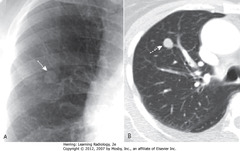
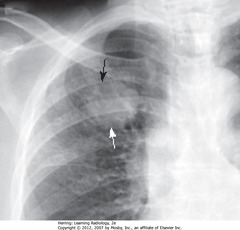
• S: 1.8 cm nodule in RUL
• Whether benign or malignant depends on lesion size, availability of prior imaging studies (show growth over time), PET scan
• Dx: adenocarcinoma of lung
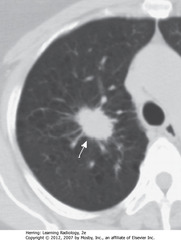
• SWA: 3.2 cm spiculated mass in RUL
• Large size and irregular margins indicate malignant process
• Dx: percutaneous biopsy revealed adenocarcinoma of lung
• Heavily calcified pulmonary nodules usually benign
• Homogenously calcified TB granuloma – common sequelae of prior, usually subclinical, TB infection (A-BCs)
• Histoplasmoma (B)
• Central/”target” calcification; may have laminated calcification – diagnostic (BA)
• CT to differentiate between calcified and noncalcified pulmonary nodule w/greater sensitivity than CXR
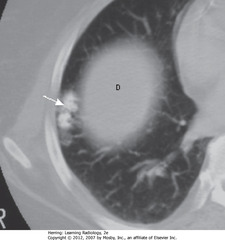
• Peripherally located tumors of disorganized lung tissue, usually contain fat and calcification on CT
• SWA: Popcorn Calcification – classical calcification of hamartoma
• Small island of soft tissue in middle of the right lung (D) – uppermost part of R Hd
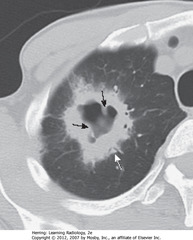
• SWA: large, cavitating neoplasm w/thick wall in RUL
• Spiculated outer margin
• SBAs: nodular internal contour of cavity
• Cavitating malignancy – this was SCC
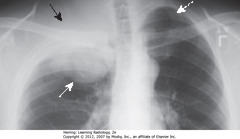
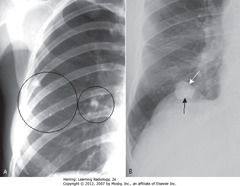
• SWA: large, soft tissue mass -R lung apex
• SBA: rib destruction
• DWA: ribs intact on normal left side
• Apical soft tissue mass with associated rib destruction classic for Pancoast or superior sulcus tumor
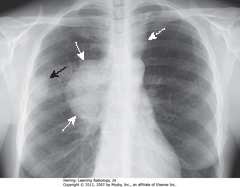
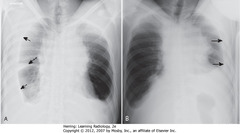
• Peripheral lung mass (SBAs)
• Evidence of ipsilateral hilar and mediastinal adenopathy (SWAs)
• Contralateral mediastinal adenopathy (DWA)
• Bronchogenic carcinoma may present w/metastatic lesions that can manifest in distant organs or in thorax itself
• Dx: adenocarcinoma of lung
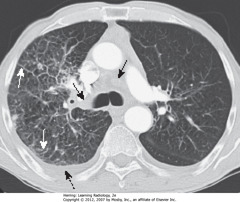
• Extensive hilar, mediastinal adenopathy from lung carcinoma (SBA)
• Prominent interstitial markings R lung
• Thick septal lines (Kerley B lines) (SWA)
• R pleural effusion (DBA)
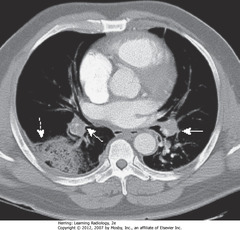
• DWA: Hampton Hump – wedge-shaped, peripheral air-space density, associated with filling defects in both
• SWAs: R and L pulmonary arteries
• Without associated emboli present, pleural-based airspace disease DDx includes PNA, lung contusion, aspiration
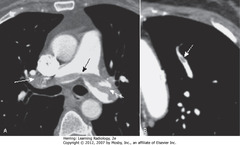
• Acute PEs appear as partial or complete filling defects centrally located w/in contrast-enhanced lumina of pulmonary arteries
• SW: Saddle embolus – large pulmonary embolus, almost completely fills both pulmonary arteries
• DWA: small, central filling defect in a more peripheral pulmonary
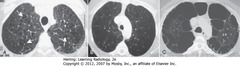
• A-SWA: CENTRIACINAR (centrilobular): focal destruction – bronchioles, central portions of acinus; assoc w/ smoking, most severe in ULs
• B: PANACINAR (panlobular) emphysema: entire alveolus distal to terminal bronchiole; most severe in lower lung zones; usually w/homozygous alpha1-antitrypsin deficiency
• C: PARASEPTAL emphysema: least common form; distal airway structures, alveolar ducts, sacs; usually subpleural, may cause PTX
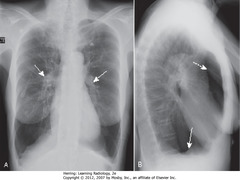
• Prominent pulmonary arteries 2° pulmonary arterial HTN (A – SWA)
• Flattened diaphragm (B – SWA)
• Increased retrosternal clear space (B-DWA)
• Hyperlucency of lungs with less than normal normal vascular markings
• CXR findings of COPD: hyperinflation, flattening of diaphragm, especially on lateral exposure
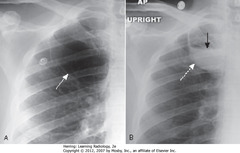
• A – SWA: several thin-walled, but air-containing, bullae in right upper lobe
• B – DWA: several weeks later, one of the bullae contains both fluid and air
• B – SBA: fluid and air in bulla
• Bullae normally contain air but can become partially or completely fluid-filled from infection or hemorrhage
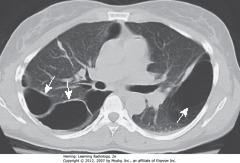
• SWA: bullae – typically contain no blood vessels but septae may appear to traverse the bulla
• Bullae measure > 1 cm in size
• They have a very thin wall, often only partially visible on CXR
• CT demonstrates them more easily – on CXR, presence often inferred by localized paucity of lung markings
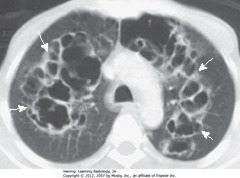
• SWAs: multiple cysts in upper lobes
• Cysts visible on CXR in 10% of patients with PCP, but far more frequently with CT scans (up to 1 in 3 patients)
• May occur in the acute or postinfective phase of disease
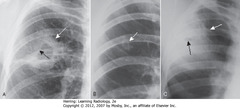
• 3 of MC cavitary lung lesions often differentiated by thickness of cavity wall, smoothness/nodularity of inner margin
• A – SWA: cavity w/thick wall (squamous cell bronchogenic carcinoma)
• A – SBA: nodular interior margin
• B – SWA: relatively thin-walled, upper lobe cavity with smooth inner margin (TB)
• C – SWA: thickened wall of staphylococcal lung abscess
• C – SBA: very small cavity with smooth inner
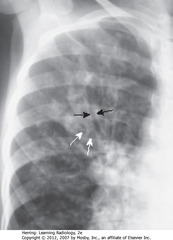
• SBA: Tram Tracks – parallel line opacities on CXR due to thickened walls of dilated bronchi
• SWA: cystic bronchiectasis – cystic lesions as large as 2 cm in diameter
• Bilateral upper lobe bronchiectasis in children highly suggestive of cystic fibrosis
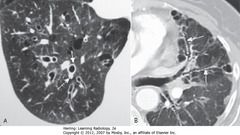
• A-SWA: Signet-Ring Sign – thickened bronchus wall, becomes larger than associated pulmonary artery (opposite of normal)
• A – DWA: associated pulmonary artery
• B – SWA: Tram Tracking (parallel line opacities from thickened walls of dilated bronchi), thickened walls, failure to taper normally
• CT is the study of choice in diagnosing bronchiectasis





